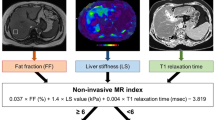
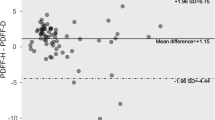
Abstract
Low-cost non-invasive diagnostic tools for staging the progression of non-alcoholic chronic liver failure from fatty liver disease to steatohepatitis are unavailable. Here, we describe the development and performance of a portable single-sided magnetic-resonance sensor for grading liver steatosis and fibrosis using diffusion-weighted multicomponent T2 relaxometry. In a diet-induced mouse model of non-alcoholic fatty liver disease, the sensor achieved overall accuracies of 92% (Cohen’s kappa, κ = 0.89) and 86% (κ = 0.78) in the ex vivo grading of steatosis and fibrosis, respectively. Localization of the measurements in living mice through frequency-dependent spatial encoding led to an overall accuracy of 87% (κ = 0.81) for the grading of steatosis. In human liver samples, the sensor graded steatosis with an overall accuracy of 93% (κ = 0.88). The use of T2 relaxometry as a sensitive measure in fully automated low-cost magnetic-resonance devices at the point of care would alleviate the accessibility and cost limits of magnetic-resonance imaging for diagnosing liver disease and assessing liver health before liver transplantation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. The raw and analysed datasets generated during the study are too large to be publicly shared, yet they are available for research purposes from the corresponding author on reasonable request.
Code availability
All code used for the analysis of data generated during the study is available from the corresponding author on reasonable request.
References
Younossi, Z. et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 15, 11–20 (2018).
Shetty, A. & Syn, W.-K. Health and economic burden of nonalcoholic fatty liver disease in the United States and its impact on veterans. Fed. Pract. 36, 14–19 (2019).
Spengler, E. K. & Loomba, R. Recommendations for diagnosis, referral for liver biopsy, and treatment of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Mayo Clin. Proc. 90, 1233–1246 (2015).
Brunt, E. M. Pathology of nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 7, 195–203 (2010).
Vernon, G., Baranova, A. & Younossi, Z. M. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 34, 274–285 (2011).
Takahashi, Y. & Fukusato, T. Histopathology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J. Gastroenterol. 20, 15539–15548 (2014).
Wattacheril, J., Issa, D. & Sanyal, A. Nonalcoholic steatohepatitis (NASH) and hepatic fibrosis: emerging therapies. Annu. Rev. Pharmacol. Toxicol. 58, 649–662 (2018).
Zhang, E. et al. Cost-utility analysis of nonalcoholic steatohepatitis screening. Eur. Radiol. 25, 3282–3294 (2015).
Tanaka, N. et al. Current status, problems, and perspectives of non-alcoholic fatty liver disease research. World J. Gastroenterol. 25, 163–177 (2019).
Wong, V. W.-S., Adams, L. A., de Lédinghen, V., Wong, G. L.-H. & Sookoian, S. Noninvasive biomarkers in NAFLD and NASH—current progress and future promise. Nat. Rev. Gastroenterol. Hepatol. 15, 461–478 (2018).
Strauss, S., Gavish, E., Gottlieb, P. & Katsnelson, L. Interobserver and intraobserver variability in the sonographic assessment of fatty liver. Am. J. Roentgenol. 189, W320–W323 (2007).
Liang, W. et al. Establishment of a general NAFLD scoring system for rodent models and comparison to human liver pathology. PLoS ONE 9, e115922 (2014).
Shen, J. et al. Non-invasive diagnosis of non-alcoholic steatohepatitis by combined serum biomarkers. J. Hepatol. 56, 1363–1370 (2012).
Krawczyk, K. et al. Adipohormones as prognostic markers in patients with nonalcoholic steatohepatitis (NASH). J. Physiol. Pharmacol. 60, 71–75 (2009).
Loomba, R. et al. Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: a prospective study. Hepatology 60, 1920–1928 (2014).
Akkaya, H. E., Erden, A., Öz, D. K., Ünal, S. & Erden, I. Magnetic resonance elastography: basic principles, technique, and clinical applications in the liver. Diagn. Interv. Radiol. 24, 328–335 (2018).
Fishbein, M. et al. Hepatic MRI for fat quantitation: its relationship to fat morphology, diagnosis, and ultrasound. J. Clin. Gastroenterol. 39, 619–625 (2005).
Taouli, B. et al. Diffusion-weighted MRI for quantification of liver fibrosis: preliminary experience. Am. J. Roentgenol. 189, 799–806 (2007).
Petitclerc, L., Sebastiani, G., Gilbert, G., Cloutier, G. & Tang, A. Liver fibrosis: review of current imaging and MRI quantification techniques. J. Magn. Reson. Imaging 45, 1276–1295 (2017).
Dulai, P. S., Sirlin, C. B. & Loomba, R. MRI and MRE for non-invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: clinical trials to clinical practice. J. Hepatol. 65, 1006–1016 (2016).
Bohte, A. E., van Werven, J. R., Bipat, S. & Stoker, J. The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur. Radiol. 21, 87–97 (2011).
Schultz, J. F., Bell, J. D., Goldstein, R. M., Kuhn, J. A. & McCarty, T. M. Hepatic tumor imaging using iron oxide MRI: comparison with computed tomography, clinical impact, and cost analysis. Ann. Surg. Oncol. 6, 691–698 (1999).
Evens, R. G. & Evens, R. G. Economic and utilization analysis of MR imaging units in the United States in 1987. Radiology 166, 27–30 (1988).
Schroeder, S. A. Magnetic resonance imaging: present costs and potential gains. Ann. Intern. Med. 102, 551–553 (1985).
Bashyam, A., Frangieh, C., Li, M. & Cima, M. J. Dehydration assessment via a non-invasive, miniature, portable magnetic resonance sensor using multicomponent T2 relaxometry. Magn. Reson. Med. 83, 1390–1404 (2020).
Bashyam, A., Li, M. & Cima, M. J. Design and experimental validation of unilateral linear Halbach magnet arrays for single-sided magnetic resonance. J. Magn. Reson. 292, 36–43 (2018).
Bush, E. C. et al. Fat-water phantoms for magnetic resonance imaging validation: a flexible and scalable protocol. J. Vis. Exp. 139, e57704 (2018).
Hines, C. D. G. et al. T1 independent, T2* corrected MRI with accurate spectral modeling for quantification of fat: validation in a fat-water-SPIO phantom. J. Magn. Reson. Imaging 30, 1215–1222 (2009).
Bernard, C. P., Liney, G. P., Manton, D. J., Turnbull, L. W. & Langton, C. M. Comparison of fat quantification methods: a phantom study at 3.0T. J. Magn. Reson. Imaging 27, 192–197 (2008).
Whitaker, C. & Casarella, J. Multiple NMR T2 relaxation values in human liver tissue. Am. J. Roentgenol. 141, 1203–1208 (1983).
Cole, W. C., Leblanc, A. D. & Jhingran, S. G. The origin of biexponential T2 relaxation in muscle water. Magn. Reson. Med. 29, 19–24 (1993).
Clapper, J. R. et al. Diet-induced mouse model of fatty liver disease and nonalcoholic steatohepatitis reflecting clinical disease progression and methods of assessment. Am. J. Physiol. Gastrointest. Liver Physiol. 305, G483–G495 (2013).
Nakamura, A. & Terauchi, Y. Lessons from mouse models of high-fat diet-induced NAFLD. Int. J. Mol. Sci. 14, 21240–21257 (2013).
Matsumoto, M. et al. An improved mouse model that rapidly develops fibrosis in non-alcoholic steatohepatitis. Int. J. Exp. Pathol. 94, 93–103 (2013).
Ikawa-Yoshida, A. et al. Hepatocellular carcinoma in a mouse model fed a choline-deficient, l-amino acid-defined, high-fat diet. Int. J. Exp. Pathol. 98, 221–233 (2017).
van Werven, J. R. et al. Assessment of hepatic steatosis in patients undergoing liver resection: comparison of US, CT, T1-weighted dual-echo MR imaging, and point-resolved 1H MR spectroscopy. Radiology 256, 159–168 (2010).
Charlton, M. et al. Fast food diet mouse: novel small animal model of NASH with ballooning, progressive fibrosis, and high physiological fidelity to the human condition. Am. J. Physiol. Gastrointest. Liver Physiol. 301, G825–G834 (2011).
Kleiner, D. E. et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41, 1313–1321 (2005).
Bonekamp, S., Torbenson, M. S. & Kamel, I. R. Diffusion-weighted magnetic resonance imaging for the staging of liver fibrosis. J. Clin. Gastroenterol. 45, 885–892 (2011).
Casanova, F., Perlo, J. & Blümich, B. Single-Sided NMR (Springer, 2011).
Casieri, C., Bubici, S. & De Luca, F. Self-diffusion coefficient by single-sided NMR. J. Magn. Reson. 162, 348–355 (2003).
Rata, D. G., Casanova, F., Perlo, J., Demco, D. E. & Blümich, B. Self-diffusion measurements by a mobile single-sided NMR sensor with improved magnetic field gradient. J. Magn. Reson. 180, 229–235 (2006).
Perlo, J., Casanova, F. & Blümich, B. Profiles with microscopic resolution by single-sided NMR. J. Magn. Reson. 176, 64–70 (2005).
Koinuma, M., Ohashi, I., Hanafusa, K. & Shibuya, H. Apparent diffusion coefficient measurements with diffusion-weighted magnetic resonance imaging for evaluation of hepatic fibrosis. J. Magn. Reson. Imaging 22, 80–85 (2005).
Eddowes, P. J. et al. Utility and cost evaluation of multiparametric magnetic resonance imaging for the assessment of non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 47, 631–644 (2018).
Skoien, R. et al. Heterogeneity of fibrosis patterns in non-alcoholic fatty liver disease supports the presence of multiple fibrogenic pathways. Liver Int. 33, 624–632 (2013).
Howlett, D. C., Drinkwater, K. J., Lawrence, D., Barter, S. & Nicholson, T. Findings of the UK national audit evaluating image-guided or image-assisted liver biopsy. Part II. Minor and major complications and procedure-related mortality. Radiology 266, 226–235 (2013).
Castéra, L., Nègre, I., Samii, K. & Buffet, C. Patient-administered nitrous oxide/oxygen inhalation provides safe and effective analgesia for percutaneous liver biopsy: a randomized placebo-controlled trial. Am. J. Gastroenterol. 96, 1553–1557 (2001).
Piccinino, F. et al. Complications following percutaneous liver biopsy: a multicentre retrospective study on 68,276 biopsies. J. Hepatol. 2, 165–173 (1986).
Seeff, L. B. et al. Complication rate of percutaneous liver biopsies among persons with advanced chronic liver disease in the HALT-C trial. Clin. Gastroenterol. Hepatol. 8, 877–883 (2010).
Rockey, D. C., Caldwell, S. H., Goodman, Z. D., Nelson, R. C. & Smith, A. D. Liver biopsy. Hepatology 49, 1017–1044 (2009).
Foster, G. R. et al. Management of chronic hepatitis C: clinical audit of biopsy based management algorithm. Brit. Med. J. 315, 453–458 (1997).
Clark, J. M., Brancati, F. L. & Diehl, A. M. Nonalcoholic fatty liver disease. Gastroenterology 122, 1649–1657 (2002).
Yano, E., Tagawa, K., Yamaoka, K. & Mori, M. Test validity of periodic liver function tests in a population of Japanese male bank employees. J. Clin. Epidemiol. 54, 945–951 (2001).
Stanković, M. N. et al. Time-dependent changes and association between liver free fatty acids, serum lipid profile and histological features in mice model of nonalcoholic fatty liver disease. Arch. Med. Res. 45, 116–124 (2014).
Linares, I., Hamar, M., Selzner, N. & Selzner, M. Steatosis in liver transplantation: current limitations and future strategies. Transplantation 103, 78–90 (2019).
Croome, K. P. et al. The impact of post-reperfusion syndrome during liver transplantation using livers with significant macrosteatosis. Am. J. Transplant. https://doi.org/10.1111/ajt.15330 (2019).
Gabrielli, M. et al. Steatotic livers. Can we use them in OLTX? Outcome data from a prospective baseline liver biopsy study. Ann. Hepatol. 11, 891–898 (2012).
Croome, K. P., Lee, D. D. & Taner, C. B. The “skinny” on assessment and utilization of steatotic liver grafts: a systematic review. Liver Transpl. 25, 488–499 (2019).
Selzner, M. & Clavien, P. A. Fatty liver in liver transplantation and surgery. Semin. Liver Dis. 21, 105–113 (2001).
Rehorn, C. & Blümich, B. Cultural heritage studies with mobile NMR. Angew. Chem. Int. Ed. Engl. 57, 7304–7312 (2018).
Dabaghyan, M. et al. A portable single-sided magnet system for remote NMR measurements of pulmonary function. NMR Biomed. 27, 1479–1489 (2014).
Colucci, L. A. et al. Fluid assessment in dialysis patients by point-of-care magnetic relaxometry. Sci. Transl. Med. 11, eaau1749 (2019).
Bruinsma, B. G. et al. Subnormothermic machine perfusion for ex vivo preservation and recovery of the human liver for transplantation. Am. J. Transplant. 14, 1400–1409 (2014).
Karimian, N. et al. Ex situ normothermic machine perfusion of donor livers. J. Vis. Exp. 2015, e52688 (2015).
Acknowledgements
We thank staff at the Koch Institute Swanson Biotechnology Center for technical support, specifically W. Huang and V. Spanoudaki at Animal Imaging and Preclinical Testing and K. Cormier at Histology; A. Warren, K. Nayak, M. Rosen, E. Adalsteinsson, S. Carrasco, K. Subramanyam, M. Cotler, E. Rousseau and K. Ramadi for discussions; staff at the New England Donor Services and the patients and families that made this study possible. This work was supported in part by the Koch Institute Support (core) grant no. P30-CA14051 from the National Cancer Institute, the National Institutes of Health grant nos. R01DK096075 and R01DK107875, and National Science Foundation (ATP-Bio 1941543). A.B. was supported by a Fannie & John Hertz Foundation Graduate Fellowship and a National Science Foundation Graduate Fellowship.
Author information
Authors and Affiliations
Contributions
A.B. constructed the portable MR sensor. A.B. and C.J.F. characterized the portable MR sensor, performed all of the experiments and wrote the manuscript. J.S. performed ex vivo experiments. A.B. prepared figures. A.B., C.J.F. and M.J.C. designed the experiments and interpreted results. A.B., C.J.F., S.R. and M.J.C. edited the manuscript. R.T.B. conducted pathology analysis of all tissues. M.J.C., D.A.A., H.Y. and K.U. supervised the research.
Corresponding author
Ethics declarations
Competing interests
A.B. and M.J.C. are inventors on a patent application (US20180306879) submitted by MIT that describes the design of the permanent magnet array within the portable MR sensor. K.U. has a financial interest in Organ Solutions, a company that is focused on developing organ preservation technology. These interests are managed by the Mass General Brigham in accordance with their conflict of interest policies.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–15, Table 1, discussion, methods and references.
Rights and permissions
About this article
Cite this article
Bashyam, A., Frangieh, C.J., Raigani, S. et al. A portable single-sided magnetic-resonance sensor for the grading of liver steatosis and fibrosis. Nat Biomed Eng 5, 240–251 (2021). https://doi.org/10.1038/s41551-020-00638-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-020-00638-0
This article is cited by
-
Single-sided magnetic resonance-based sensor for point-of-care evaluation of muscle
Nature Communications (2024)
-
Molecular imaging: design mechanism and bioapplications
Science China Chemistry (2023)
-
Quantifying Liver Fat Using a Low-Field Unilateral MR System
Applied Magnetic Resonance (2023)